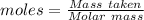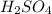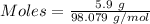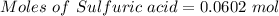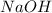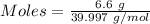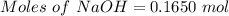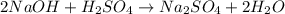Chemistry, 05.11.2019 00:31 safiyabrowne7286
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sulfate (na2so4) and liquid water (h2o). what is the theoretical yield of water formed from the reaction of 5.9 g of sulfuric acid and 6.6 g of sodium hydroxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

You know the right answer?
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sul...
Questions


Chemistry, 15.04.2021 20:10

Mathematics, 15.04.2021 20:10

World Languages, 15.04.2021 20:10

History, 15.04.2021 20:10

Chemistry, 15.04.2021 20:10










Mathematics, 15.04.2021 20:10

Mathematics, 15.04.2021 20:10


Mathematics, 15.04.2021 20:10

Mathematics, 15.04.2021 20:10