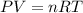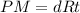Chemistry, 05.11.2019 00:31 Imamdiallo18
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is the density of krypton gas in this container at stp? at stp one mole of any gas occupies 22.4 l. (r = 0.08206 l • atm/k • mol) atomic mass of krypton is 83.8 amu. select one: a. 0.0561 g/l b. 0.0176 g/l c. 3.74 g/l d. 181 g/l e. 1.78 g/l

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is th...
Questions

Social Studies, 15.07.2019 05:40


Business, 15.07.2019 05:40



History, 15.07.2019 05:40

Social Studies, 15.07.2019 05:40

Social Studies, 15.07.2019 05:40

History, 15.07.2019 05:40


Chemistry, 15.07.2019 05:40

Social Studies, 15.07.2019 05:40

History, 15.07.2019 05:40

Biology, 15.07.2019 05:40

History, 15.07.2019 05:40


History, 15.07.2019 05:40

Mathematics, 15.07.2019 05:40

English, 15.07.2019 05:40

History, 15.07.2019 05:40