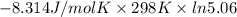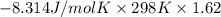Chemistry, 05.11.2019 00:31 jessemartinez1
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.109 atm. once equilibrium has been established, it is found that pc = 0.047 atm. what is δg° for this reaction (in kj/mol) at 25°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.1...
Questions





Mathematics, 24.02.2021 22:30

Physics, 24.02.2021 22:30

Mathematics, 24.02.2021 22:30


Mathematics, 24.02.2021 22:30

English, 24.02.2021 22:30

Mathematics, 24.02.2021 22:30


Mathematics, 24.02.2021 22:30

Mathematics, 24.02.2021 22:30

Mathematics, 24.02.2021 22:30

Physics, 24.02.2021 22:30

Physics, 24.02.2021 22:30

Physics, 24.02.2021 22:30

SAT, 24.02.2021 22:30

Mathematics, 24.02.2021 22:30

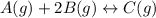
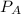 = 0.109 atm,
= 0.109 atm, 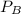 = 0.109 atm,
= 0.109 atm,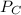 = 0.109 atm
= 0.109 atm![[0.109 + (2 \times 0.062)]](/tpl/images/0359/5284/f1aca.png) atm
atm 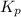 as follows.
as follows.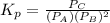
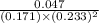
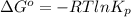
 as follows.
as follows.