
Chemistry, 05.11.2019 01:31 bunbun2913
A. the concentration of oh- ions in a sample of seawater is 5.0 x 10-6m. calculate the concentration of h+ ions and determine the ph and poh of this solution. is it neutral, acidic, or basic? b. the concentration of h+ ions in a sample of lemon juice is 2.5 x 10-3m. calculate the concentration of oh- ions and determine the ph and poh of this solution. is it neutral, acidic, or basic?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
A. the concentration of oh- ions in a sample of seawater is 5.0 x 10-6m. calculate the concentration...
Questions


English, 19.02.2021 03:00

Mathematics, 19.02.2021 03:00


Mathematics, 19.02.2021 03:00



Mathematics, 19.02.2021 03:00

English, 19.02.2021 03:00


Geography, 19.02.2021 03:00


Biology, 19.02.2021 03:00




Mathematics, 19.02.2021 03:00



Mathematics, 19.02.2021 03:00

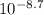 = [H⁺] = 2.0 x 10⁻⁹ M
= [H⁺] = 2.0 x 10⁻⁹ M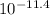 = [OH⁻] = 4.0 x 10⁻¹² M
= [OH⁻] = 4.0 x 10⁻¹² M

