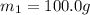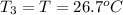Asilver block, initially at 58.4∘c, is submerged into 100.0 g of water at 25.0∘c in an insulated container. the final temperature of the mixture upon reaching thermal equilibrium is 26.7∘c. the specific heat capacities for water and silver are cs, water=4.18j/(g⋅∘c) and cs, silver=0.235j/(g⋅∘c).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
Asilver block, initially at 58.4∘c, is submerged into 100.0 g of water at 25.0∘c in an insulated con...
Questions


Mathematics, 15.04.2021 22:20

Mathematics, 15.04.2021 22:20

History, 15.04.2021 22:20





Business, 15.04.2021 22:20

Mathematics, 15.04.2021 22:20




English, 15.04.2021 22:20

Spanish, 15.04.2021 22:20

Mathematics, 15.04.2021 22:20

Advanced Placement (AP), 15.04.2021 22:20

Mathematics, 15.04.2021 22:20



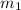

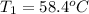
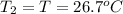 =
=