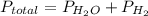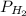Chemistry, 05.11.2019 02:31 jitosfc916
If the vapor pressure of water ( ) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer (
) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer ( ) was 757.3 mm hg, what is the partial pressure of h₂ gas (
) was 757.3 mm hg, what is the partial pressure of h₂ gas ( ) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for
) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for  )
)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
If the vapor pressure of water ([tex]p_{h_2o}[/tex]) at 20.0°c is 17.5 mm hg, and the atmospheric pr...
Questions

Health, 27.01.2021 02:20

English, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20



History, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20



Mathematics, 27.01.2021 02:20

English, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20




Mathematics, 27.01.2021 02:20


English, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20

Engineering, 27.01.2021 02:20