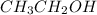Chemistry, 05.11.2019 02:31 devbar3416
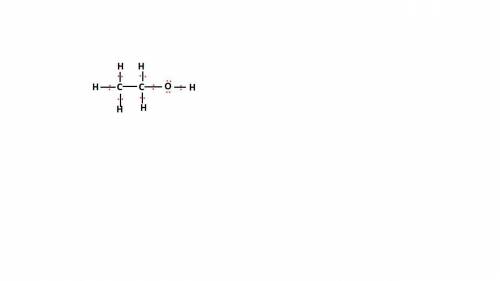
Write the lewis structure for ethanol (ch3ch2oh), the alcohol found in alcoholic beverages, then answer the following questions: 1. how many valence electrons does this alcohol have? 2. how many bonded electrons does this alcohol have? 3. how many lone pairs of electrons does this alcohol have? 4. how many single bonds does this alcohol have?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3


Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
Write the lewis structure for ethanol (ch3ch2oh), the alcohol found in alcoholic beverages, then ans...
Questions



English, 28.06.2019 23:20



Mathematics, 28.06.2019 23:20


Mathematics, 28.06.2019 23:20





Mathematics, 28.06.2019 23:20




Mathematics, 28.06.2019 23:20

Health, 28.06.2019 23:20