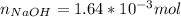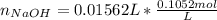Chemistry, 05.11.2019 02:31 powellmom5
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissolved in 10.0 ml of ethyl alcohol and 25.0 ml of di water. the titration of this solution with 0.1052 m naoh required 15.62 ml to reach the phenolphthalein endpoint. determine the moles of naoh that reacted with the acetylsalicylic acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissol...
Questions








Computers and Technology, 17.09.2019 20:00








Biology, 17.09.2019 20:00