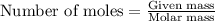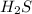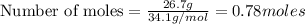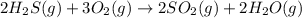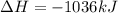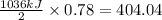Hydrogen sulfide burns form sulfur dioxide:
2h2s(g) +3o2(g) → 2so2(g) + 2h2o(g) δh= -10...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
Questions

French, 22.04.2021 04:20


Business, 22.04.2021 04:20

Mathematics, 22.04.2021 04:20


English, 22.04.2021 04:20

Social Studies, 22.04.2021 04:20


Computers and Technology, 22.04.2021 04:20


Biology, 22.04.2021 04:20

Mathematics, 22.04.2021 04:20

Mathematics, 22.04.2021 04:20


Mathematics, 22.04.2021 04:20

Mathematics, 22.04.2021 04:20




Mathematics, 22.04.2021 04:20