
Chemistry, 05.11.2019 03:31 paytonpaige22
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chemical equation for the reaction. (hint: water-soluble alcl3 is the stable chloride of aluminum.) (b) calculate the mass of pure aluminum that will furnish 10.0 l hydrogen at a pressure of 0.750 atm and a temperature of 30.0°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

You know the right answer?
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chem...
Questions

Mathematics, 02.03.2021 19:40




Law, 02.03.2021 19:40



Mathematics, 02.03.2021 19:40


Mathematics, 02.03.2021 19:40

Chemistry, 02.03.2021 19:40

History, 02.03.2021 19:40


English, 02.03.2021 19:40



Mathematics, 02.03.2021 19:40

Mathematics, 02.03.2021 19:40


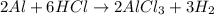
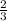 mole of aluminum is consumed.
mole of aluminum is consumed.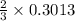 mole of aluminum is consumed.
mole of aluminum is consumed.

