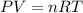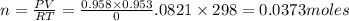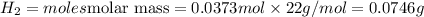Chemistry, 05.11.2019 04:31 babyface1686
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). the reaction between the acid and the zinc is as follows: 2h+(aq)+zn(s)→h2(g)+zn2+(aq). when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c was 0.953 l at a total pressure of 752 mmhg .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions



Mathematics, 16.10.2019 01:50

English, 16.10.2019 01:50

History, 16.10.2019 01:50


History, 16.10.2019 01:50

Mathematics, 16.10.2019 01:50

Mathematics, 16.10.2019 01:50


Mathematics, 16.10.2019 01:50


Chemistry, 16.10.2019 01:50


Mathematics, 16.10.2019 01:50

Mathematics, 16.10.2019 02:00

Chemistry, 16.10.2019 02:00



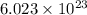 of particles.
of particles.