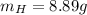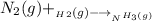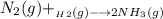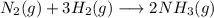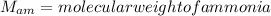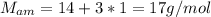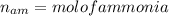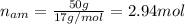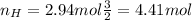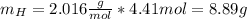Nitrogen and hydrogen react together to form ammonia according to the equation: (g) + (g) → (g) (unbalanced)balance the equation, and determine how many grams of hydrogen would be required to form 50.0 g of ammonia, assuming there is sufficient nitrogen available.4.46 g5.94 g4.81 g8.91 gnot enough information

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

You know the right answer?
Nitrogen and hydrogen react together to form ammonia according to the equation: (g) + (g) → (g) (unb...
Questions

History, 03.08.2019 04:00




History, 03.08.2019 04:00






Mathematics, 03.08.2019 04:00

Mathematics, 03.08.2019 04:00

Mathematics, 03.08.2019 04:00


Mathematics, 03.08.2019 04:00




Mathematics, 03.08.2019 04:00

Mathematics, 03.08.2019 04:00