
Chemistry, 05.11.2019 04:31 angelapegues20097
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxygen gas at standard temperature. on heating, the iron and oxygen react according to the balanced reaction below. 4fe(s) + 3o2(g) → 2fe2o3(s) after the reaction vessel is cooled, and assuming the reaction goes to completion, what pressure of oxygen remains?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxyg...
Questions





Social Studies, 04.11.2020 22:40

English, 04.11.2020 22:40


Social Studies, 04.11.2020 22:40

Mathematics, 04.11.2020 22:40

History, 04.11.2020 22:40



History, 04.11.2020 22:40

Mathematics, 04.11.2020 22:40

History, 04.11.2020 22:40



History, 04.11.2020 22:40

Mathematics, 04.11.2020 22:40

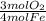 = 0.3765 mol O₂
= 0.3765 mol O₂

