
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.0690 m naoh . the pka values for aspartic acid are 1.990 , 3.900 , and 10.002 , corresponding to the α-carboxylic acid group, the β-carboxylic acid group, and the amino group, respectively. calculate the ph at the first equivalence point of this titration.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.06...
Questions

Biology, 30.09.2019 11:00


Mathematics, 30.09.2019 11:00


English, 30.09.2019 11:00




History, 30.09.2019 11:00

Biology, 30.09.2019 11:00

History, 30.09.2019 11:00


History, 30.09.2019 11:00


Mathematics, 30.09.2019 11:00

Social Studies, 30.09.2019 11:00

Mathematics, 30.09.2019 11:00


Geography, 30.09.2019 11:00

English, 30.09.2019 11:00

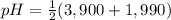 = 2,945
= 2,945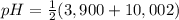 = 6,951
= 6,951

