Chemistry, 05.11.2019 05:31 sainijasdeep27
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases.
bulb a: 200. ml of kr(g) at 190. torr
bulb b: 400. ml of h2s(g) at 1.00 atm
bulb c: 1.00 l of n2(g) at 75.994 kpa
after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions

Social Studies, 24.11.2021 09:40

English, 24.11.2021 09:40

Biology, 24.11.2021 09:40

Chemistry, 24.11.2021 09:40

Mathematics, 24.11.2021 09:40

Mathematics, 24.11.2021 09:40

Mathematics, 24.11.2021 09:40



Mathematics, 24.11.2021 09:40




Mathematics, 24.11.2021 09:40


Mathematics, 24.11.2021 09:40

Geography, 24.11.2021 09:40

Mathematics, 24.11.2021 09:40


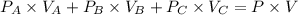
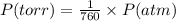
 P (atm)
P (atm)
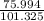 atm
atm