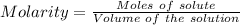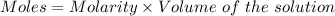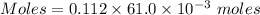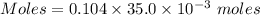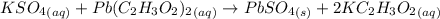A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acetate solution and the following precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)? 2kc2h3o2(aq)+pbso4(s)
the solid pbso4 is collected, dried, and found to have a mass of 0.997g .
determine the limiting reactant, the theoretical yield, and the percent yield.
part a.
identify the limiting reactant.
kc2h3o2
pb(c2h3o2)2
k2so4
pbso4
part b.
determine the theoretical yield.
mass of pbso4 =
part c.
determine the percent yield=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acet...
Questions

Mathematics, 26.10.2020 18:10

Mathematics, 26.10.2020 18:10

Mathematics, 26.10.2020 18:10


Social Studies, 26.10.2020 18:10


Mathematics, 26.10.2020 18:10

History, 26.10.2020 18:10

Mathematics, 26.10.2020 18:10



Arts, 26.10.2020 18:10

Health, 26.10.2020 18:10

French, 26.10.2020 18:10

Mathematics, 26.10.2020 18:10




English, 26.10.2020 18:10

Mathematics, 26.10.2020 18:10