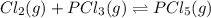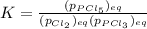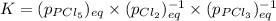Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole fraction of the indicated substance in its phase, which we have so far assumed equal to unity (one) for essentially pure phases.)
cl2(g) + pcl3(g)↔pcl5(g))
choose from the list below and enter the letters alphabetical order. (e. g. ah)
(a) (pcl2)eq
(b) (ppcl3)eq
(c) (ppcl5)eq
(d) (pcl2)eq-1
(e) (ppcl3)eq-1
(f) (ppcl5)eq-1
(g) (pcl2)eq2
(h) (ppcl3)eq3
(i) (ppcl5)eq5
(j) (pcl2)eq-2
(k) (ppcl3)eq-3
(l) (ppcl5)eq-5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole frac...
Questions

Mathematics, 12.05.2020 03:57

Physics, 12.05.2020 03:57



Mathematics, 12.05.2020 03:57

English, 12.05.2020 03:57




History, 12.05.2020 03:57



Mathematics, 12.05.2020 03:57



Medicine, 12.05.2020 03:57

History, 12.05.2020 03:57



 .
.