
Chemistry, 05.11.2019 22:31 realoneree
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(i) nitrate and chromium(iii) bromide are combined. 3agno3 (aq) + crbr3 (aq) --> 3agbr (s) + cr(no3)3 (aq) write the balanced net ionic equation for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions


Mathematics, 24.05.2020 20:57




Mathematics, 24.05.2020 20:57

Mathematics, 24.05.2020 20:57

History, 24.05.2020 20:57


Mathematics, 24.05.2020 20:57

Mathematics, 24.05.2020 20:57


Mathematics, 24.05.2020 20:57


History, 24.05.2020 20:57

Mathematics, 24.05.2020 20:57

English, 24.05.2020 20:57


Mathematics, 24.05.2020 20:57

History, 24.05.2020 20:57


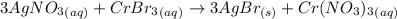
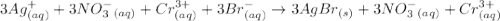
 are the spectator ions.
are the spectator ions.


