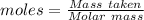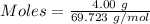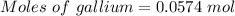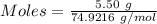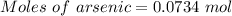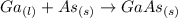Chemistry, 05.11.2019 22:31 Princess14321
Molten gallium reacts with arsenic to form the semiconductor, gallium arsenide, gaas, used in light emitting diodes and solar cells: ga(l) + as(s) → gaas(s) if 4.00 g of gallium is reacted with 5.50 g of arsenic how many grams of the excess reactant are left at the end of the reaction?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Molten gallium reacts with arsenic to form the semiconductor, gallium arsenide, gaas, used in light...
Questions


Mathematics, 22.01.2021 19:10

Mathematics, 22.01.2021 19:10


Mathematics, 22.01.2021 19:10


English, 22.01.2021 19:10

Social Studies, 22.01.2021 19:10



Mathematics, 22.01.2021 19:10

Arts, 22.01.2021 19:10

Mathematics, 22.01.2021 19:10

History, 22.01.2021 19:10



English, 22.01.2021 19:10

Health, 22.01.2021 19:10


Mathematics, 22.01.2021 19:10