
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is there in 1 mole of electrons? how much charge is there in 1 mole of hydrogen ions (h+). remember that hydrogen consists of one electron and one proton, so hydrogen ions are just protons.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1


Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is...
Questions

Health, 05.05.2020 17:39



Mathematics, 05.05.2020 17:39


Health, 05.05.2020 17:39

English, 05.05.2020 17:39





Computers and Technology, 05.05.2020 17:39



Mathematics, 05.05.2020 17:39


Mathematics, 05.05.2020 17:39



Arts, 05.05.2020 17:39

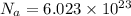
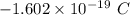
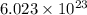 electrons =
electrons = 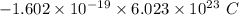 = -96488.46 C
= -96488.46 C

