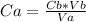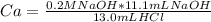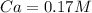Chemistry, 06.11.2019 01:31 realoneree
In a titration experiment, 13.0 ml of an aqueous hcl solution was titrated with 0.2 m naoh solution. the equivalence point in the titration was reached when 11.1 ml of the naoh solution was added. what is the molarity of the hcl solution? hcl molarity

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
In a titration experiment, 13.0 ml of an aqueous hcl solution was titrated with 0.2 m naoh solution....
Questions

History, 28.09.2019 12:10


World Languages, 28.09.2019 12:10




Mathematics, 28.09.2019 12:10


History, 28.09.2019 12:10

World Languages, 28.09.2019 12:10

Advanced Placement (AP), 28.09.2019 12:10

Mathematics, 28.09.2019 12:10

Biology, 28.09.2019 12:10


Social Studies, 28.09.2019 12:10

Mathematics, 28.09.2019 12:10




Mathematics, 28.09.2019 12:10