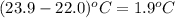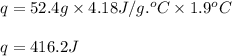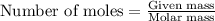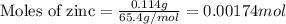Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(aq) h2(g) when 0.114 g of zn(s) is combined with enough hcl to make 52.4 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.0 ∘c to 23.9 ∘c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(a...
Questions




Physics, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50



Mathematics, 18.11.2020 17:50

Advanced Placement (AP), 18.11.2020 17:50






Mathematics, 18.11.2020 17:50

Geography, 18.11.2020 17:50


Mathematics, 18.11.2020 17:50

Chemistry, 18.11.2020 17:50

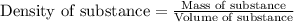
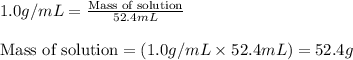
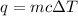
 = change in temperature =
= change in temperature =