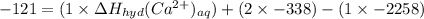Chemistry, 06.11.2019 02:31 wirchakethan23
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium chloride is -2258 kj/mol and the heat of hydration of a chloride ion is -338 kj/mol calculate the heat of hydration of a calcium ion.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2


Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium ch...
Questions





Arts, 23.01.2022 14:00

Biology, 23.01.2022 14:00



History, 23.01.2022 14:00



French, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00


English, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00




Social Studies, 23.01.2022 14:00

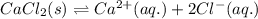
![\Delta H_{sol}=[1mol\times \Delta H_{hyd}(Ca^{2+})_{aq.}]+[2mol\times \Delta H_{hyd}(Cl^{-})_{aq.}]-[1mol\times U(CaCl_{2})_{s}]](/tpl/images/0361/2975/af20b.png)
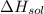 is heat of solution,
is heat of solution, 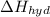 is heat of hydration and U represents lattice energy.
is heat of hydration and U represents lattice energy.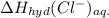 = -338 kJ/mol and
= -338 kJ/mol and 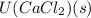 = -2258 kJ/mol
= -2258 kJ/mol