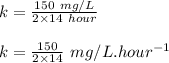Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the concentration of chemical x drops from 150 mg/l to 75 mg/l in 14 hours.
(a) what is the half-life?
(b) what is the decay constant if the reaction is first order?
(c) what is the decay constant if the reaction is zero order?
(d) how would you determine whether the reaction is zero or first order? if you had a set of concentration

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3
You know the right answer?
Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the...
Questions


Mathematics, 05.05.2020 23:07

Mathematics, 05.05.2020 23:07

Computers and Technology, 05.05.2020 23:07











Physics, 05.05.2020 23:07



English, 05.05.2020 23:07

Business, 05.05.2020 23:07

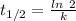
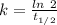
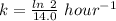
![t_{1/2}=\frac{[A]_0}{2k}](/tpl/images/0361/2925/15a8b.png)
![[A]_0](/tpl/images/0361/2925/7075c.png) is the initial concentration = 150 mg/L
is the initial concentration = 150 mg/L