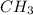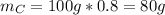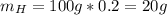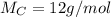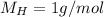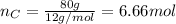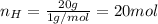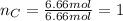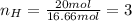Chemistry, 06.11.2019 04:31 chloebaby8
Acompound is 80.0% carbon and 20.0% hydrogen by mass. assume you have a 100.-g sample of this compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
Acompound is 80.0% carbon and 20.0% hydrogen by mass. assume you have a 100.-g sample of this compou...
Questions



Social Studies, 12.08.2020 06:01







Social Studies, 12.08.2020 06:01