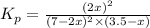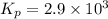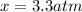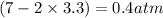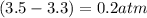Chemistry, 06.11.2019 04:31 PlsHelpMeh3401
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a starting mixture with 7.0 atm no and 3.5 atm cl2. (hint: kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)
2 no(g) + cl2(g) --> 2 nocl(g)kp = 2.9 ✕ 103 at 149°c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a...
Questions

Mathematics, 21.09.2021 14:00


Social Studies, 21.09.2021 14:00

English, 21.09.2021 14:00

Spanish, 21.09.2021 14:00




English, 21.09.2021 14:00



Mathematics, 21.09.2021 14:00



Computers and Technology, 21.09.2021 14:00

History, 21.09.2021 14:00

Social Studies, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00


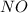 ,
,  , and
, and  in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.
in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.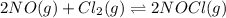
![K_p=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0361/4899/09f8c.png)