
Chemistry, 06.11.2019 06:31 maggie123433
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid solution and 50.03 g of 1.0 m sodium hydroxide solution to the calorimeter. both solutions have an initial temperature of 19.9 oc, and the final temperature after addition is 26.8 oc. what is the molar enthalpy of neutralization, in units of kj/mol? assume that: the calorimeter is completely insulated the heat capacity of the empty calorimeter is the heat capacity of the aluminum cup: 0.903 j g-1 oc-1. the density of the two solutions is the same as that of water: 1.00 g/ml. the heat capacity of the two solutions is the same as that of water: 4.184 j g-1 oc-1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid soluti...
Questions

Mathematics, 31.01.2020 10:01

Mathematics, 31.01.2020 10:01



Mathematics, 31.01.2020 10:01

Mathematics, 31.01.2020 10:01


Mathematics, 31.01.2020 10:01

Mathematics, 31.01.2020 10:01



Mathematics, 31.01.2020 10:01


Mathematics, 31.01.2020 10:01



English, 31.01.2020 10:01


Health, 31.01.2020 10:01

Chemistry, 31.01.2020 10:01

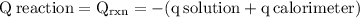
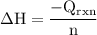
![\rm \DeltaH=-[\dfrac{- 3412.6007}{0.05003}]\\\\\Delta H=\:68211.087\dfrac{J}{mole} =68.211\dfrac{kJ}{mol}](/tpl/images/0361/7451/7b811.png)


