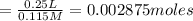Chemistry, 06.11.2019 07:31 mmcdaniels46867
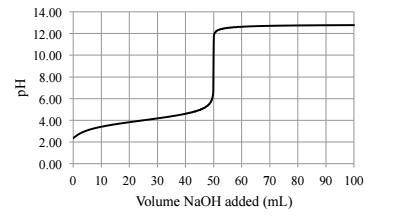
A25.0 ml sample of a solution of a monoprotic acid is titrated with a 0.115 m naoh solution. the titration curve above was obtained. the concentration of the monoprotic acid is about mol/l.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
A25.0 ml sample of a solution of a monoprotic acid is titrated with a 0.115 m naoh solution. the tit...
Questions


Biology, 11.10.2020 01:01

Biology, 11.10.2020 01:01

Computers and Technology, 11.10.2020 01:01



Mathematics, 11.10.2020 01:01



Mathematics, 11.10.2020 01:01



English, 11.10.2020 01:01


Mathematics, 11.10.2020 01:01




Health, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01