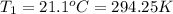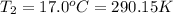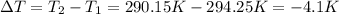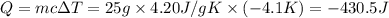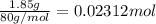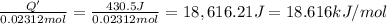Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the endothermic reaction: nh4no3 (s) --> nh4+ (aq) + no3- (aq) 1.85g ammonium nitrate is added to water in a calorimeter. the total solution (water and ammonium nitrate) is 25.0g. the heat capacity of the calorimeter is ccal=45.0 j/k. the initial temperature of the solution is 21.1c. the final temperature of the solution is 17.0c. assume cs of the solution is 4.20 j/(g k) what is δhrxn per mol of the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water sep...
Questions


Mathematics, 23.10.2020 15:50





Geography, 23.10.2020 15:50












Computers and Technology, 23.10.2020 15:50

Chemistry, 23.10.2020 15:50