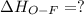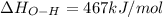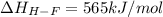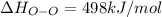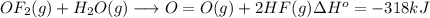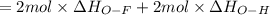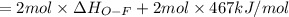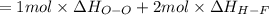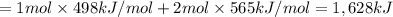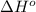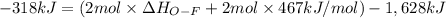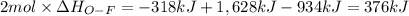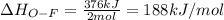Chemistry, 07.11.2019 01:31 jenlicavoli
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy of the o–f bond using the standard enthalpy of reaction and the bond energy data provided. just enter a number (no units). of2(g) + h2o(g) \longrightarrow⟶ o=o(g) + 2hf(g) \deltaδh° = –318 kj bond: o–h o=o h–f bond energy (kj/mol): 467 498 565

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy...
Questions

Mathematics, 03.12.2019 07:31


Biology, 03.12.2019 07:31


History, 03.12.2019 07:31



Mathematics, 03.12.2019 07:31


Mathematics, 03.12.2019 07:31




Mathematics, 03.12.2019 07:31

Business, 03.12.2019 07:31


Mathematics, 03.12.2019 07:31


Mathematics, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31