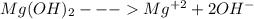Chemistry, 07.11.2019 01:31 shimmerandshine1
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x 10-4. this leads to an [oh-] = 2.8 x 10-4 (i. e., a poh=3.55), thus a ph of 10.45. given the aforementioned information and resulting calculations, comment on the solubility of mg(oh)2 at ph values: 1) less than 10.45 and, 2) greater than 10.45

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x...
Questions






Biology, 05.04.2021 16:00



Spanish, 05.04.2021 16:00

Biology, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00

Biology, 05.04.2021 16:00





Mathematics, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00