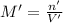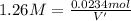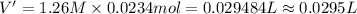Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion.
cr3+(aq)+3naoh(aq) → cr(oh)3(s)+3na+(aq)
if you had a 0.600 l solution containing 0.0130 m of cr3+(aq), and you wished to add enough 1.26 m naoh(aq) toprecipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.
express the volume to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

You know the right answer?
Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reacti...
Questions

Social Studies, 23.06.2019 02:30



Mathematics, 23.06.2019 02:30




Health, 23.06.2019 02:30


Health, 23.06.2019 02:30






Advanced Placement (AP), 23.06.2019 02:30




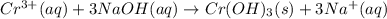
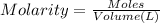
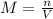
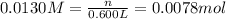
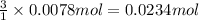 of NaOH
of NaOH