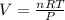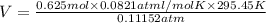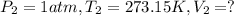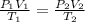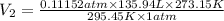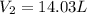Chemistry, 07.11.2019 01:31 KariSupreme
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that follows. a 15.0 mg piece of solid magnesium was reacted completely with hydrochloric acid in a reaction flask with a volume of 135 ml. the temperature of the reaction was 22.3 °c and the pressure of the gas produced by the reaction was 11.3 kpa. calculate the volume of hydrogen gas that would have formed in this reaction had it been conducted under standard temperature and pressure conditions (use the combined gas law). use the volume you have just determined, along with the number of moles of hydrogen gas that would have formed from 15.0 mg of magnesium reactant, to calculate the molar volume of this gas at stp

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that f...
Questions




Computers and Technology, 04.01.2020 04:31







Social Studies, 04.01.2020 04:31



Social Studies, 04.01.2020 04:31


Social Studies, 04.01.2020 04:31




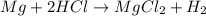
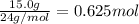
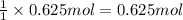 of hydrogen gas.
of hydrogen gas.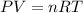 (ideal gas equation)
(ideal gas equation)