
Chemistry, 07.11.2019 01:31 crosales102
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or base is added. suppose that you have 155 ml of a buffer that is 0.180 m in both formic acid (hcooh) and its conjugate base (hcoo−) . calculate the maximum volume of 0.450 m hcl that can be added to the buffer before its buffering capacity is lost.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or ba...
Questions


Computers and Technology, 22.01.2020 00:31

English, 22.01.2020 00:31



English, 22.01.2020 00:31

Arts, 22.01.2020 00:31


Mathematics, 22.01.2020 00:31



Mathematics, 22.01.2020 00:31

English, 22.01.2020 00:31

Mathematics, 22.01.2020 00:31

History, 22.01.2020 00:31

Mathematics, 22.01.2020 00:31

Health, 22.01.2020 00:31


Mathematics, 22.01.2020 00:31

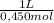 = 0,0253L ≡ 25,3 mL that are the maximum volume you can add.
= 0,0253L ≡ 25,3 mL that are the maximum volume you can add.

