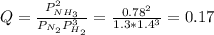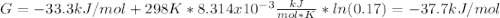In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ at 298k for this reaction is −33.3kj/mol. the value of δg at 298k for a reaction mixture that consists of 1.3atmn2, 1.4atmh2, and 0.78atmnh3 is kj/mol. in the haber process, ammonia is synthesized from nitrogen and hydrogen: at for this reaction is . the value of at for a reaction mixture that consists of , , and is . −4.42×103 −76.6 −37.7 −5.7 −2.13 × 103

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ a...
Questions

Physics, 20.11.2019 22:31



Biology, 20.11.2019 22:31

Mathematics, 20.11.2019 22:31

English, 20.11.2019 22:31

Mathematics, 20.11.2019 22:31

History, 20.11.2019 22:31

Social Studies, 20.11.2019 22:31

Mathematics, 20.11.2019 22:31

History, 20.11.2019 22:31


Engineering, 20.11.2019 22:31






English, 20.11.2019 22:31

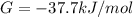
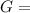 Δ
Δ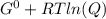
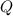 is computed via the law of mass action:
is computed via the law of mass action: