
Chemistry, 07.11.2019 02:31 whitwelllife
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium) must be added to an aqueous solution of 0.100m pyridinium (assume the solution volume is constant) to make a buffer that is designed to keep the ph at 4.7?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium)...
Questions


Spanish, 02.06.2021 23:10

English, 02.06.2021 23:10

Mathematics, 02.06.2021 23:10

Mathematics, 02.06.2021 23:10


Mathematics, 02.06.2021 23:10




Chemistry, 02.06.2021 23:10



English, 02.06.2021 23:10

Biology, 02.06.2021 23:10





Computers and Technology, 02.06.2021 23:10

![pH=pK_a+log\frac{[Conjugate\ base]}{[weak\ acid]}](/tpl/images/0363/0377/c0611.png)
![pH=pK_a+log\frac{[Py]}{PyH^+]}](/tpl/images/0363/0377/17718.png)
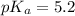
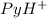 (Pyridinium)=0.100 M
(Pyridinium)=0.100 M![pH=pK_a+log\frac{[Py]}{PyH^+]}\\4.7=5.2 log\frac{[Py]}{0.100}](/tpl/images/0363/0377/de0ef.png)
![4.7-5.2=log\frac{[Py]}{0.100} \\-0.5=log\frac{[Py]}{0.100}\\\frac{[Py]}{0.100}=antilog -0.5\\\frac{[Py]}{0.100}=0.316](/tpl/images/0363/0377/5a83b.png)
![\frac{[Py]}{0.100} =0.316](/tpl/images/0363/0377/cf365.png)


