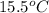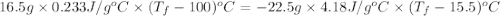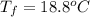Chemistry, 07.11.2019 04:31 genesisheaven1
Acoin dealer, offered a rare silver coin, suspected that it might be a counterfeit nickel copy. the dealer heated the coin, which weighed 16.5 g to 100°c in boiling water and then dropped the hot coin into 22.5 g of water at t = 15.5°c in an insulated coffee-cup, and measured the rise in temperature. if the coin was really made of silver, what would the final temperature of the water be (in °c)? (for nickel, s = 0.445 j/g°c; for silver, s = 0.233 j/g°c )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1
You know the right answer?
Acoin dealer, offered a rare silver coin, suspected that it might be a counterfeit nickel copy. the...
Questions










Business, 20.12.2019 03:31










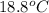
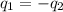
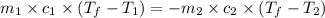
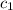 = specific heat of silver =
= specific heat of silver = 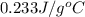
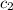 = specific heat of water =
= specific heat of water = 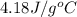
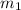 = mass of silver coin = 16.5 g
= mass of silver coin = 16.5 g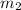 = mass of water = 22.5 g
= mass of water = 22.5 g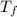 = final temperature of water = ?
= final temperature of water = ?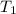 = initial temperature of silver coin =
= initial temperature of silver coin = 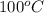
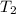 = initial temperature of water =
= initial temperature of water =