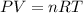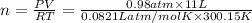Chemistry, 07.11.2019 05:31 mckleinrivero
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determine the percent composition of the alloy. the scientist takes a 13.039 g sample of the alloy and reacts it with concentrated hcl . the reaction converts all of the aluminum and zinc in the alloy to aluminum chloride and zinc chloride in addition to producing hydrogen gas. the copper does not react with the hcl . upon completion of the reaction, a total of 11 l of hydrogen gas was collected at a pressure of 744 torr and a temperature of 27.0 °c . additionally, 2.761 g of unreacted copper is recovered. calculate the mass of hydrogen gas formed from the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
You know the right answer?
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determ...
Questions

Computers and Technology, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10


Mathematics, 19.01.2021 22:10




Social Studies, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10


Mathematics, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10


Mathematics, 19.01.2021 22:10



English, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10