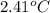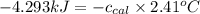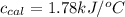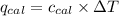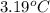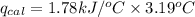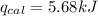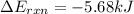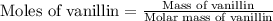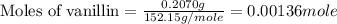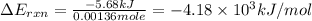The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. calculate the heat capacity of this calorimeter. (the energy released by combustion of benzoic acid is 26.42 kj/g.)
kj/°c
a 0.2070 g sample of vanillin (c8h8o3) is then burned in the same calorimeter, and the temperature increases by 3.19°c. what is the energy of combustion per gram of vanillin?
kj/g
what is the energy of combustion per mole of vanillin?
kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. c...
Questions


Social Studies, 31.08.2019 12:00





Computers and Technology, 31.08.2019 12:00


History, 31.08.2019 12:00

English, 31.08.2019 12:00


Mathematics, 31.08.2019 12:00

Biology, 31.08.2019 12:00

History, 31.08.2019 12:00


Social Studies, 31.08.2019 12:00

History, 31.08.2019 12:00

Health, 31.08.2019 12:00


English, 31.08.2019 12:10

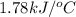
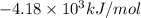
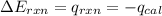
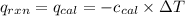
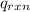 = heat released by the reaction = -4.293 kJ
= heat released by the reaction = -4.293 kJ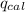 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter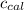 = specific heat of calorimeter = ?
= specific heat of calorimeter = ? = change in temperature =
= change in temperature =