
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml of solution. to the solution, 35.435.4 ml of 0.02190.0219 m kcl kcl is added, resulting in the complete precipitation of the silver ash agcl agcl . the excess cl−cl− added to the solution is then back titrated with a standard 0.02190.0219 m agno3 agno3 solution. a total of 14.3314.33 ml of the standard nano3 agno3 solution is required to reach the silver chromate end point in the mohr method. calculate the percent silver in the piece of metal.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml...
Questions



Geography, 07.04.2020 02:30

Geography, 07.04.2020 02:30

History, 07.04.2020 02:30







Biology, 07.04.2020 02:30


Mathematics, 07.04.2020 02:31




Mathematics, 07.04.2020 02:31


Mathematics, 07.04.2020 02:31

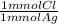 = 0.314 mmol Cl⁻
= 0.314 mmol Cl⁻

