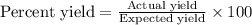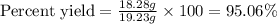Chemistry, 07.11.2019 07:31 ZaNiyahlove4711
Calculate the % yield if the amount of alum obtained was 18.28 g and the expected amount was 19.93 g.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1
You know the right answer?
Calculate the % yield if the amount of alum obtained was 18.28 g and the expected amount was 19.93 g...
Questions

Mathematics, 01.09.2020 02:01


Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01



Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01


Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01


Mathematics, 01.09.2020 02:01

Mathematics, 01.09.2020 02:01

History, 01.09.2020 02:01



Business, 01.09.2020 02:01