Chemistry, 07.11.2019 21:31 BigGirlsTheBest
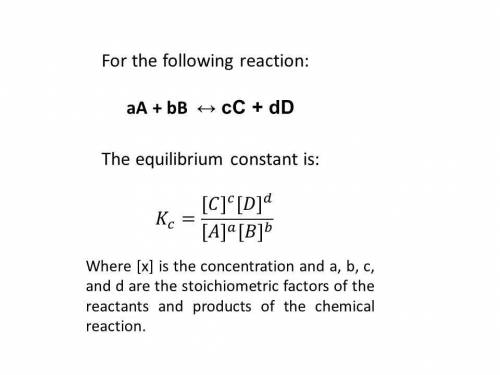
At a certain temperature, the equilibrium constant, kc, kc, for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(g)kc=53.3 h2(g)+i2(g)↽−−⇀2hi(g)kc=53.3 at this temperature, 0.400 mol h20.400 mol h2 and 0.400 mol i20.400 mol i2 were placed in a 1.00 l container to react. what concentration of hihi is present at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

You know the right answer?
At a certain temperature, the equilibrium constant, kc, kc, for this reaction is 53.3. h2(g)+i2(g)↽−...
Questions

Biology, 08.12.2020 01:40



Mathematics, 08.12.2020 01:40

Chemistry, 08.12.2020 01:40

History, 08.12.2020 01:40


Chemistry, 08.12.2020 01:40

Chemistry, 08.12.2020 01:40



Computers and Technology, 08.12.2020 01:40






Mathematics, 08.12.2020 01:40


Mathematics, 08.12.2020 01:40

![K_{c} = \frac{[HI]^{2}}{[H_{2}][I_{2}]} = 53.3](/tpl/images/0364/2800/9c8ef.png) (2)
(2)![[H_{2}] = [I_{2}] = \frac{0.400 mol}{1 L} = 0.400 mol/L](/tpl/images/0364/2800/3a013.png)

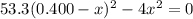
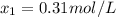
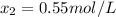
![[HI] = 2x = 2*0.31 mol/L = 0.62 mol/L](/tpl/images/0364/2800/ce8eb.png)
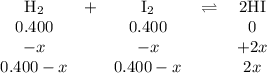
![K_{\text{c}} = \dfrac{\text{[HI]$^{2}$}}{\text{[H$_{2}$][I$_2$]}} = \dfrac{(2x)^{2}}{(0.400 - x)^{2}} = 53.3\\\\\begin{array}{rcl}\dfrac{(2x)^{2}}{(0.400 - x)^{2}} &=& 53.3\\ \dfrac{2x }{0.400 - x} & = & 7.301\\\\2x & = & 7.301(0.400 - x)\\2x & = & 2.920 - 7.301x\\9.301x & = & 2.920\\x & = & \dfrac{2.920}{9.301}\\\\x & = & \mathbf{0.3140}\\\end{array}](/tpl/images/0364/2800/cfef9.png)