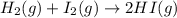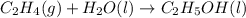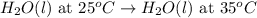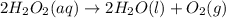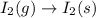Chemistry, 08.11.2019 00:31 salmanderabdi12
Determine whether the entropy, δs, increases or decreases for the following reactions. in some cases, more information is needed. mark those as "more information needed". h2 (g) + i2 (g) → 2hi (g). c2h4 (g) + h2o (l) → c2h5oh (l) h2o (l) at 25oc → h2o (l) at 35 oc 2h2o2 (aq) → 2 h2o (l) + o2 (g) i2 (g) → i2 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Determine whether the entropy, δs, increases or decreases for the following reactions. in some cases...
Questions




Mathematics, 06.04.2020 00:49

Mathematics, 06.04.2020 00:49


Mathematics, 06.04.2020 00:49





Geography, 06.04.2020 00:49


Medicine, 06.04.2020 00:50


Mathematics, 06.04.2020 00:50

Mathematics, 06.04.2020 00:50

Biology, 06.04.2020 00:50

Mathematics, 06.04.2020 00:50

Mathematics, 06.04.2020 00:50