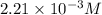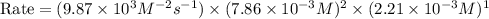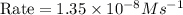Chemistry, 08.11.2019 00:31 giavanleer14
2no + o22 no2 is second order in no and first order in o2. complete the rate law for this reaction in the box below. use the form , where '1' is understood for m, n (don't enter 1) and concentrations taken to the zero power do not appear. rate = in an experiment to determine the rate law, the rate constant was determined to be 9.87×103 m-2s-1. using this value for the rate constant, the rate of the reaction when [no] = 7.86×10-3 m and [o2] = 2.21×10-3 m would be ms-1.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
2no + o22 no2 is second order in no and first order in o2. complete the rate law for this reaction i...
Questions

Mathematics, 07.02.2021 23:00






Mathematics, 07.02.2021 23:00

Spanish, 07.02.2021 23:00

Arts, 07.02.2021 23:00


Mathematics, 07.02.2021 23:10

Mathematics, 07.02.2021 23:10



Mathematics, 07.02.2021 23:10


World Languages, 07.02.2021 23:10

Mathematics, 07.02.2021 23:10

Mathematics, 07.02.2021 23:10

Health, 07.02.2021 23:10

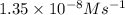
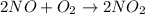
![\text{Rate}=k[NO]^a[O_2]^b](/tpl/images/0364/5919/1903b.png)
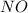 = 2
= 2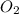 = 1
= 1![\text{Rate}=k[NO]^2[O_2]^1](/tpl/images/0364/5919/3eb46.png)
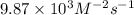
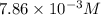
![[O_2]](/tpl/images/0364/5919/b0db0.png) = concentration of
= concentration of