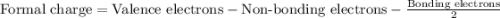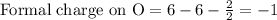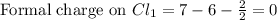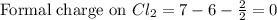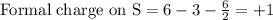Chemistry, 08.11.2019 01:31 briannaalvarado256
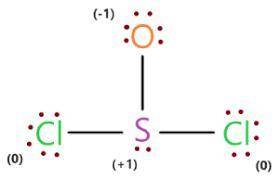
Write a lewis structure that obeys the octet rule for socl2 (s is the central atom) and assign formal charges to each atom. draw the molecule by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons and formal charges.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1


Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

You know the right answer?
Write a lewis structure that obeys the octet rule for socl2 (s is the central atom) and assign forma...
Questions


Biology, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01


Biology, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01





History, 28.08.2020 21:01

Physics, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01


Business, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01

 is shown below.
is shown below.