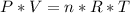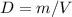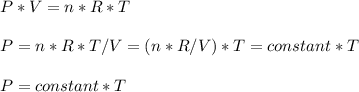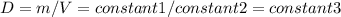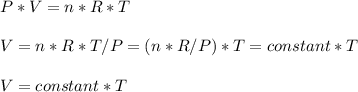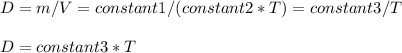Chemistry, 08.11.2019 03:31 kamkam5791
Consider two different containers, each filled with of . one of the containers is rigid and has constant volume. the other container is flexible (like a balloon) and is capable of changing its volume to keep the external pressure and internal pressure equal to each other. if you raise the temperature in both containers, what happens to the pressure and density of the gas inside each container? assume a constant external pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Consider two different containers, each filled with of . one of the containers is rigid and has cons...
Questions





Arts, 19.10.2020 07:01



Mathematics, 19.10.2020 07:01




Computers and Technology, 19.10.2020 07:01



English, 19.10.2020 07:01

English, 19.10.2020 07:01

Engineering, 19.10.2020 07:01