
Chemistry, 08.11.2019 06:31 kaziyahf2006
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when placed in a sealed container. the complete combustion of the mixture to carbon dioxide gas, co2(g) , and water vapor, h2o(g) , was achieved by adding exactly enough oxygen gas, o2(g) , to the container. the pressure of the product mixture in the sealed container is 2.378 atm . calculate the mole fraction of methane in the initial mixture, assuming the temperature and volume remain constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

You know the right answer?
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when plac...
Questions

French, 27.08.2021 06:40

Physics, 27.08.2021 06:40

Mathematics, 27.08.2021 06:40


Mathematics, 27.08.2021 06:40



Mathematics, 27.08.2021 06:40



Physics, 27.08.2021 06:40



Biology, 27.08.2021 06:40

English, 27.08.2021 06:40

Computers and Technology, 27.08.2021 06:40

Mathematics, 27.08.2021 06:40

Mathematics, 27.08.2021 06:40

English, 27.08.2021 06:40

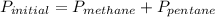
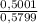 = 0,8624
= 0,8624

