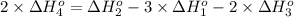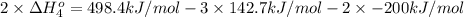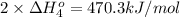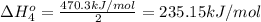Chemistry, 08.11.2019 07:31 destinystanley3794
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol for the standard enthalpy of formation of ozone and o2(g) → 2 o(g) ∆h ◦ = +498.4 kj/mol no(g) + o3(g) → no2(g) + o2(g) ∆h◦ = −200 kj/mol remember the definition of the standard enthalpy of formation of a substance.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol...
Questions


Mathematics, 15.01.2021 01:30


Mathematics, 15.01.2021 01:30

History, 15.01.2021 01:30

History, 15.01.2021 01:30

History, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30


Mathematics, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30

Chemistry, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30


Mathematics, 15.01.2021 01:30



English, 15.01.2021 01:30

English, 15.01.2021 01:30

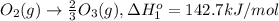 ..[1]
..[1]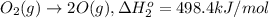 ..[2]
..[2]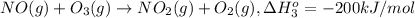 ..[3]
..[3]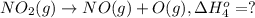 ..[4]
..[4]