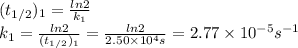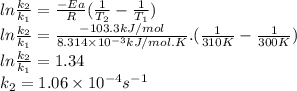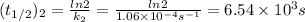For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k...

Chemistry, 08.11.2019 22:31 alexciamartinez05
For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kj/mol. what is the half-life at 310 k? (hint: use rate law expression to determine the reaction order → solve for k1 at 300 k using the corresponding half-life expression → use two-point arrhenius equation to solve for k2 at 310 k → use the half-life expression again to solve for half-life at 310 k)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
Questions


SAT, 28.07.2019 22:40

Chemistry, 28.07.2019 22:40


Mathematics, 28.07.2019 22:40

Mathematics, 28.07.2019 22:40


Arts, 28.07.2019 22:40

Social Studies, 28.07.2019 22:40

Arts, 28.07.2019 22:40

Arts, 28.07.2019 22:40



Arts, 28.07.2019 22:40

Spanish, 28.07.2019 22:40

Arts, 28.07.2019 22:40

Spanish, 28.07.2019 22:40


Arts, 28.07.2019 22:40

Arts, 28.07.2019 22:40

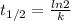
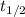 is the half-life
is the half-life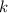 is the rate constant
is the rate constant