
Chemistry, 08.11.2019 23:31 astultz309459
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a thermos flask. when the reaction is complete, the temperature is 27.8°c. assuming that the solutions have the same heat capacity as pure water, compute the heat released (in kj).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


You know the right answer?
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a...
Questions


Biology, 28.01.2020 01:31



Mathematics, 28.01.2020 01:31



History, 28.01.2020 01:31



History, 28.01.2020 01:31


Mathematics, 28.01.2020 01:31

Mathematics, 28.01.2020 01:31

Advanced Placement (AP), 28.01.2020 01:31


Social Studies, 28.01.2020 01:31



Mathematics, 28.01.2020 01:31


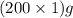 = 200 g
= 200 g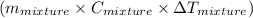
 represents change in temperature
represents change in temperature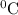 )
)![[400g\times 4.186J.g^{-1}.^{0}\textrm{C}^{-1}\times(27.8-21.0)^{0}\textrm{C} ]](/tpl/images/0366/2490/9c7ad.png) =
= 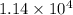 J=11.4 kJ
J=11.4 kJ

